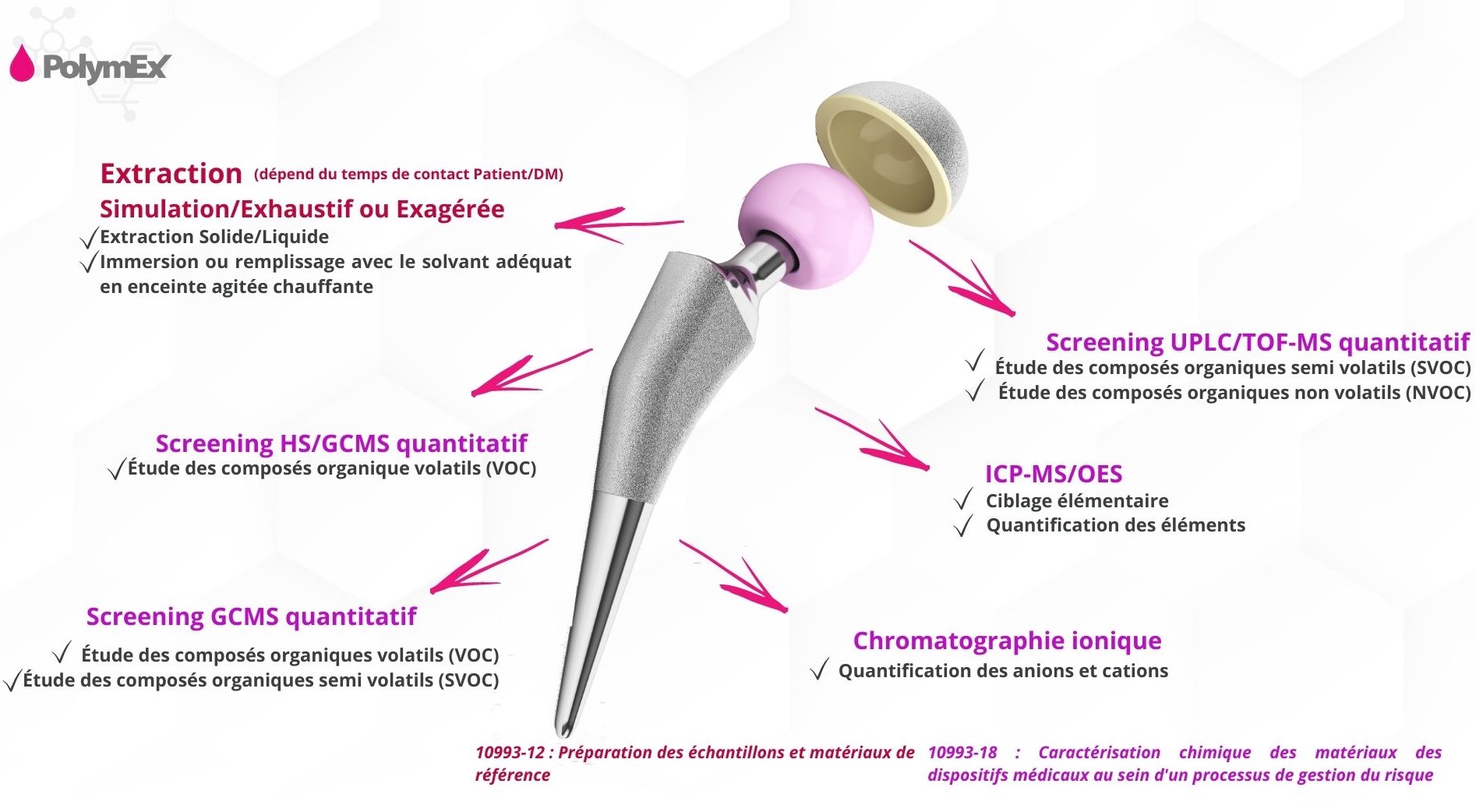
The ISO 10993 standard defines 3 types of extraction, to be preferred according to the contact classes (devices with limited contact, devices with prolonged contact, devices with long-term contact)
1 / Simulated Extraction :
An extraction with use simulation is performed in order to estimate the type and quantity of substances assumed to be released by a medical device during its clinical use.
2 / Exaggerated Extraction :
Extraction intended to result in the release of a greater number or greater amount of chemical constituents than the amount produced under clinical conditions of use.
3 / Exhaustive Extraction :
Extraction in several stages carried out so that the quantity of extracted material found in a subsequent extraction stage represents less than 10% of that detected, by gravimetric analysis (or by any other means), during the initial extraction stage.
This last standard on which Polymex works allows the characterization and chemical quantification of extractables from medical devices by scanning a very wide spectrum of extractables: organic extractables, VOC ; Organic extractables , SVOC; Organic extractables , NVOC Elemental extractables
This characterization of extractables makes it possible in particular to obtain the information essential for carrying out the toxicological evaluation according to the ISO 10993-17 standard .

 EN
EN
 FR
FR