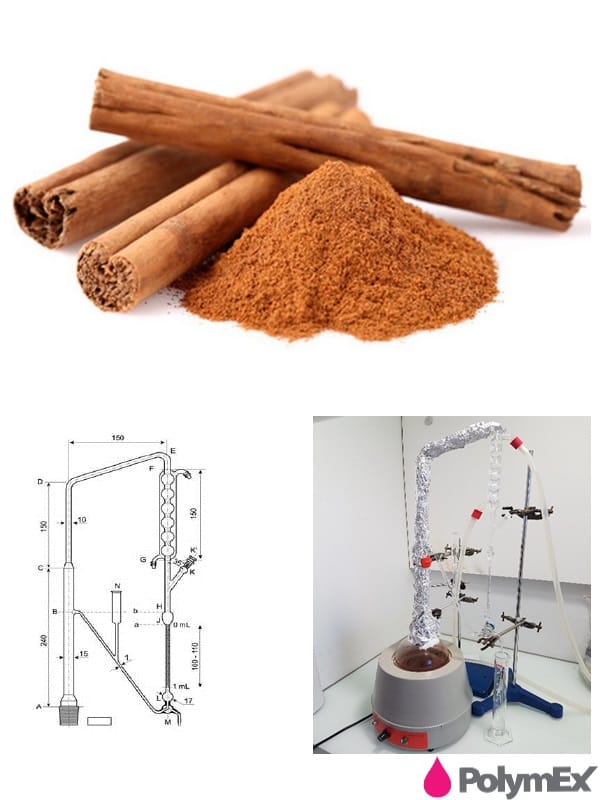
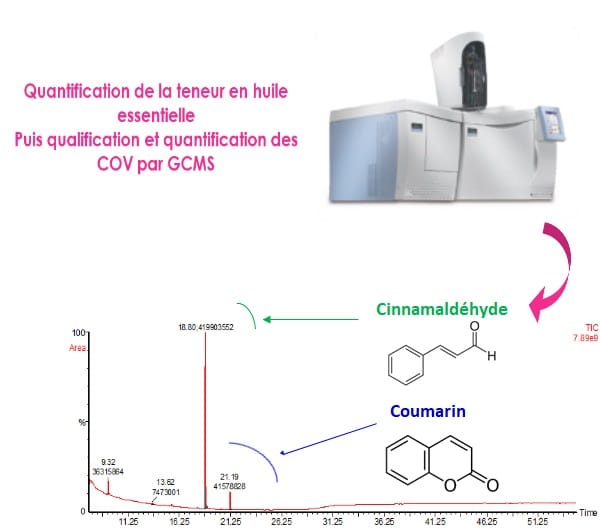
Determination of essential oil content according to the European Pharmacopoeia 9.0
The European Pharmacopoeia (also known by the diminutive Ph. Eur. ) Is the legal and scientific benchmark for pharmacopoeial standards in Europe. A unique work on quality control of medicines, it contributes to ensuring access to quality medicines.
Which products are affected by the Pharmacopoeia?
All producers of medicines and / or substances for pharmaceutical use must therefore apply this quality standard in order to be able to market their products in the States signatory to the Convention (38 European countries and used in more than a hundred countries).

 EN
EN
 FR
FR